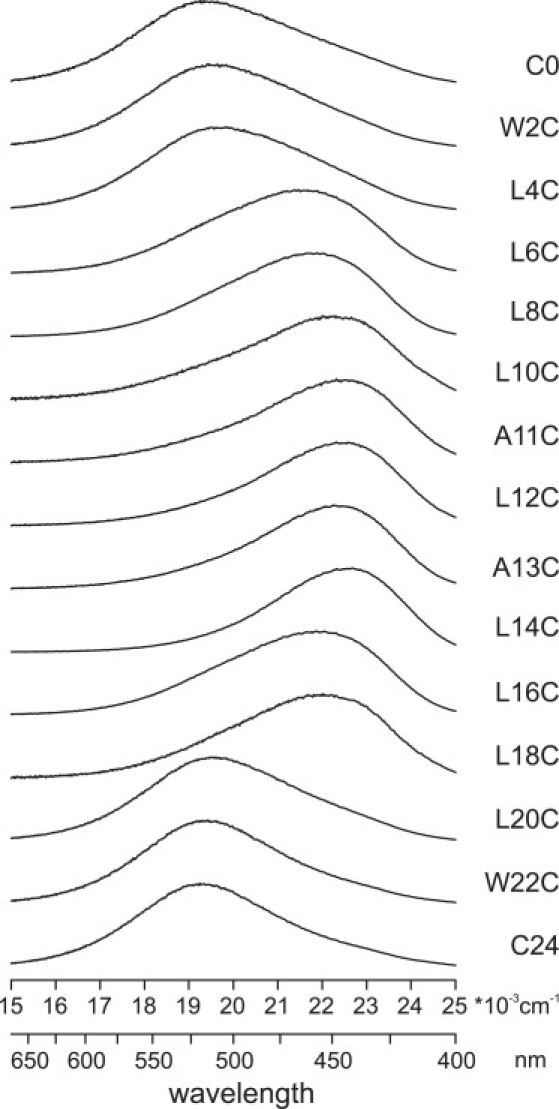
In this research the membrane orientation of a tryptophan-flanked model peptide, WALP23, was decided by utilizing peptides that have been labeled at completely different positions alongside the sequence with the environmentally delicate fluorescent label BADAN.
The fluorescence properties, reflecting the native polarity, have been used to find out the lean and rotation angles of the peptide primarily based on a perfect alpha-helix model. For WALP23 inserted in dioleoylphosphatidylcholine (DOPC), an estimated tilt angle of the helix with respect to the bilayer regular of 24 levels +/- 5 levels was obtained.
When the peptides have been inserted into bilayers with completely different acyl chain lengths or containing completely different concentrations of ldl cholesterol, small adjustments in tilt angle have been noticed as response to hydrophobic mismatch, whereas the rotation angle gave the impression to be impartial of lipid composition.
In all circumstances, the lean angles have been considerably bigger than these beforehand decided from (2)H NMR experiments, supporting current recommendations that the comparatively lengthy timescale of (2)H NMR measurements could lead to an underestimation of tilt angles on account of partial motional averaging.
It is concluded that though the fluorescence method has a slightly low decision and restricted accuracy, it may be used to resolve the discrepancies noticed between earlier (2)H NMR experiments and molecular-dynamics simulations.
High-resolution probing of native conformational adjustments in proteins by the use of a number of labeling: unfolding and self-assembly of human carbonic anhydrase II monitored by spin, fluorescent, and chemical reactivity probes.
Two completely different spin labels, N-(1-oxyl-2,2,5,5-tetramethyl-3-pyrrolidinyl)iodoacetamide (IPSL) and (1-oxyl-2,2,5,5-tetramethylpyrroline-3-methyl) methanethiosulfonate (MTSSL), and two completely different fluorescent labels 5-((((2-iodoacetyl)amino)-ethyl)amino)naphtalene-1-sulfonic acid (IAEDANS) and 6-bromoacetyl-2-dimetylaminonaphtalene (BADAN), have been connected to the launched C79 in human carbonic anhydrase (HCA II) to probe native structural adjustments upon unfolding and aggregation.
HCA II unfolds in a multi-step method with an intermediate state populated between the native and unfolded states. The spin label IPSL and the fluorescent label IAEDANS reported on a substantial change in mobility and polarity at each unfolding transitions at a distance of 7.4-11.2 A from the spine of place 79.
The shorter and much less versatile labels BADAN and MTSSL revealed much less pronounced spectroscopic adjustments within the native-to-intermediate transition, 6.6-9.zero A from the spine. At intermediate guanidine (Gu)-HCl concentrations the prevalence of soluble however irreversibly aggregated oligomeric protein was recognized from refolding experiments. At roughly 1 M Gu-HCl the aggregation was discovered to be primarily full.
The dimension and construction of the aggregates might be diverse by altering the protein focus. EPR measurements and line-shape simulations along with fluorescence lifetime and anisotropy measurements supplied a image of the self-assembled protein as a disordered protein construction with a illustration of each compact as nicely as dynamic and polar environments on the website of the molecular labels.
This means that a partially folded intermediate of HCA II self-assembles by each native unfolding and intermolecular docking of the intermediates vicinal to place 79. The aggregates have been decided to be 40-90 A in diameter relying on the experimental circumstances and spectroscopic method used.