Profiles of lipid-water bilayer dynamics have been decided from picosecond time-resolved fluorescence spectra of membrane-embedded BADAN-labeled M13 coat protein.
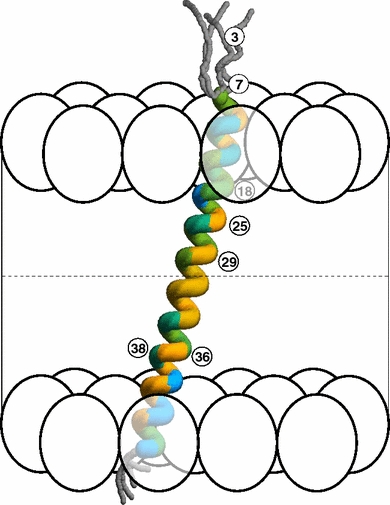
For this function, the protein was labeled at seven key positions. This locations the label at well-defined places from the water part to the middle of the hydrophobic acyl chain area of a phospholipid model membrane, offering us with a nanoscale ruler to map membranes.
Analysis of the time-resolved fluorescence spectroscopic knowledge offers the attribute time fixed for the twisting movement of the BADAN label, which is delicate to the native flexibility of the protein-lipid surroundings. In addition, we receive details about the mobility of water molecules at the membrane-water interface.
The outcomes present an unprecedented nanoscale profiling of the dynamics and distribution of water in membrane techniques. This data provides clear proof that the precise barrier of membranes for ions and aqueous solvents is positioned at the area of carbonyl teams of the acyl chains.
High affinity binding of amyloid β-peptide to calmodulin: Structural and purposeful implications.
Amyloid β-peptides (Aβ) are a main hallmark of Alzheimer’s illness (AD) and their neurotoxicity develop with cytosolic calcium dysregulation. On the different hand, calmodulin (CaM), a protein which performs a main multifunctional function in neuronal calcium signaling, has been proven to be concerned in the regulation of non-amyloidogenic processing of amyloid β precursor protein (APP).
Using fluorescent 6-bromoacetyl-2-dimethylaminonaphthalene derivatives of CaM, Badan-CaM, and human amyloid β(1-42) HiLyte™-Fluor555, we present in this work that Aβ binds with excessive affinity to CaM by the neurotoxic Aβ25-35 area. In addition, the affinity of Aβ for calcium-saturated CaM conformation is roughly 20-fold greater than for CaM conformation in the absence of calcium (apo-CaM).
Moreover, the worth of Kd of 0.98 ± 0.11 nM obtained for Aβ1-42 dissociation from CaM saturated by calcium factors out that CaM is one of the mobile targets with highest affinity for neurotoxic Aβ peptides.
A significant purposeful consequence of Aβ-CaM interplay is that it slowdowns Aβ fibrillation. The novel and excessive affinity interplay between calmodulin and Aβ proven in this work opens a yet-unexplored gateway to additional perceive the neurotoxic impact of Aβ in completely different neural cells and in addition to handle the potential of calmodulin and calmodulin-derived peptides as therapeutic brokers in AD.