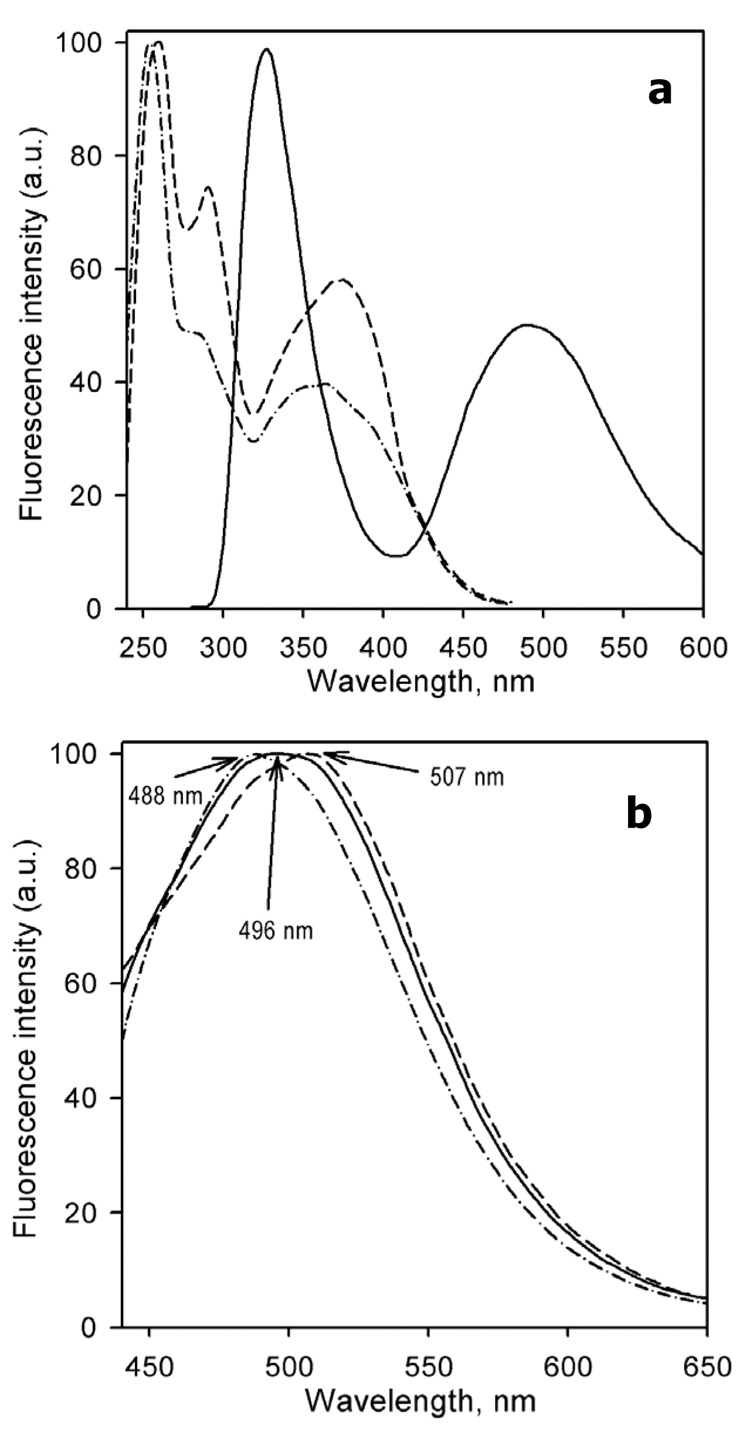
Design of a partially cysteine-depleted C98S/C239S/C377S/C468A cytochrome P450 3A4 mutant designated CYP3A4(C58,C64) allowed site-directed incorporation of thiol-reactive fluorescent probes into alpha-helix A. The website of modification was recognized as Cys-64 with the assistance of CYP3A4(C58) and CYP3A4(C64), every bearing just one accessible cysteine. Changes within the fluorescence of CYP3A4(C58,C64) labeled with 6-(bromoacetyl)-2-(dimethylamino)naphthalene (BADAN), 7-(diethylamino)-3-(4′-maleimidylphenyl)-4-methylcoumarin (CPM),…